A Step to the Table
On November 26, 1837, English analytic chemist John Newlands was born. During his working life, the Chemical and Physical Sciences were abuzz with researchers and experimenters trying to get a handle on the atomic structure of molecules and whatever else that might be discovered about atoms and their makeup.
Newlands noticed that the atomic weights of elements seem to group in a pattern. He came up with a set of seven “families” in which each element seemed to increase by seven or a multiple of seven. At the eighth point, he could represent a new family or line of elements next to the preceding column. He devised a Periodic Table of Elements, arranging the ones he knew about, and called his work the Law of Octaves.
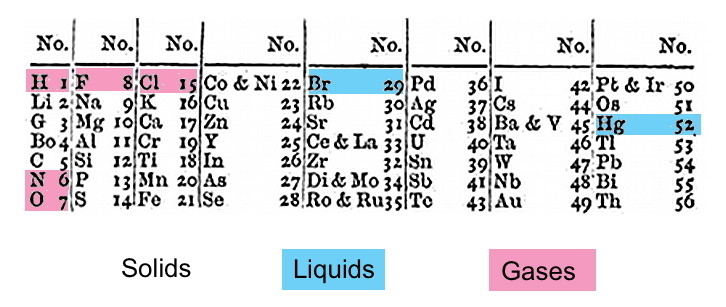
Great observation?
Here’s the kicker. Newlands did not have the latest data from international conferences on corrected atomic weights. Surprisingly, although elements were being written up with frequency, Newlands did not seem to leave space in his table for new discoveries. Perhaps he saw in his arrangement a reflection of octaves in Western music. If that is the case, then he fell into the trap of fitting data into a pattern rather than discovering a pattern into which data arranges itself. However, he did represent the ongoing international effort to find order in the basic chemical building blocks of matter.
Newlands submitted his paper for publication to the Chemical Society [Royal Society of Chemistry today]. His work was ridiculed and his paper was not published.
Elsewhere and unknown to each other, another scientist, Dmitri Mendeleev, was also working out patterns. A few years after Newlands’ Law of Octaves was rejected, Mendeleev published his Periodic Table that arranged the elements in ascending order of atomic weight, grouped by similarity of chemical properties, and accommodating future discoveries. Lother Meyer published his table much like Mendeleev’s. The Royal Society awarded Mendeleev and Meyer its Davy Medal for their periodic relationship of atomic weights.
Five years later, after fighting for recognition of his pre-existing periodic table, without assistance from the Chemical Society, Newlands received the Davy Medal for his discovery of the periodic law of the chemical elements. Today, Newlands’ work is recognized by contemporary scientists at the Royal Society of Chemistry as an historic step in the evolution of the Periodic Table.
B Bondar / Real World Content Advantage